CKD – the basics (for health professionals)
This article is largely written for health professionals. We will now go through the basics of CKD and its management.
Definition
- An estimated or measured glomerular filtration rate (GFR) of <60 mL/min – that is present for at least three months with or without evidence of kidney damage; or,
- Evidence of kidney damage with or without decreased GFR – that is present for at least three months, as evidenced by: albuminuria (protein in urine), haematuria (blood in urine, after exclusion of urological causes), structural abnormalities (e.g. on renal ultrasound) or pathological abnormalities (e.g. on kidney biopsy).
Note. In other words, in some milder cases of CKD, there is evidence of kidney damage but normal renal function (normal GFR/creatinine; see below).
When considering CKD, it is important to consider the whole of the urinary tract, and the heart. The kidneys receive 20% of cardiac output (the second largest recipient of blood, after the liver).
Mild forms of CKD exist in 10% of the population. However, 1 in 100 of these patients progress towards end-stage renal failure (ESRF) – i.e. 1 in 1ooo of the population. Therefore an average GP will have 2 patients with ESRF ‘on their books’. This is why a nephrologist (and linked specialist nurses) should be ESRF patients’ primary carer.
The inability of the kidneys to carry out their normal function leads to:
- Fluid overload – and a brittle fluid state, i.e. ERSF patients can become hypo- and hypo-volaemic quickly, sometimes in minutes
- Metabolic acidosis
- Disturbance in bone biochemistry
- Electrolyte abnormalities (including hyperkalaemia).
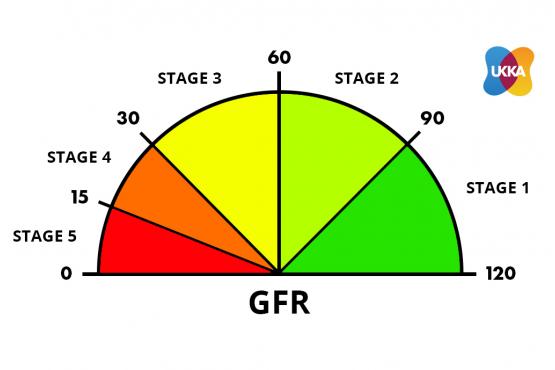
Classification of CKD (CKD stage/eGFR classification)
Courtesy of UKKA
The diagnosis of CKD is largely based on a classification of its severity, based on eGFR (estimated glomerular filtration rate) – the higher the eGFR, the better. Normal eGFR in humans is 90-120 mls/min. The eGFR is proportional to reciprocal of serum creatinine (normal range 60-120 mcmol/L) – i.e. the lower the creatinine, the higher is the GFR, the better.
The CKD classification has 5 grades. See the diagram above.
CKD1+2 = risk factors for CKD (eGFR 60-120 mls/min; with evidence of kidney damage) – no symptoms
CKD3 = mild CKD (eGFR 30-59 mls/min) – no or mild symptoms
CKD4 = moderate CKD (eGFR 15-29 mls/min) – moderate symptoms
CKD5 = severe CKD (kidney failure; eGFR < 15 mls/min) – severe symptoms (require dialysis or a transplant).
– CKD3B is worse than CKD3A
– CKD4 means the patient may require dialysis or a kidney transplant one day
– CKD5 means the patient may require dialysis, or a kidney transplant, or supportive care (no dialysis).
Note. But, the big BUT. A minor structural difference (that is not necessary a disease) is enough to lead to a diagnosis of CKD, with normal renal function. An example of this issue is microhaematuria which affects 10% of the normal population and hence is not necessarily a disease. In other words, CKD tends to overdiagnose kidney disease.
The role of the nephrologist is to decide:
a. does this patient have significant renal disease?; and
b. if so, what is the precise tissue diagnosis (cause(s))?
Note 1. After that, they do not need to control every aspect of the patients care. If discharged, the GP/practice nurse needs a clear management plan, and know how to involve the nephrologist again, rapidly if necessary. If not discharged, much of the care can be delivered by a renal specialist nurse, dietitian or pharmacist (best is a combination of them).
Note 2. CKD is different from Acute Kidney Injury (AKI), which is rapid onset kidney failure with different causes and treatments. This comes on over hours or days. It is not the focus of this article.
GFR, urinary albumin:creatinine ratio (uACR) and prognosis
CKD is further classified based on eGFR and the level of proteinuria. This helps to risk stratify patients; with a worse prognosis based on a lower eGFR, and a higher level of proteinuria (uACR)
Thus patients are classified as ‘G1-G5’, based on the eGFR, and ‘A1-A3’ based on the uACR as detailed below:
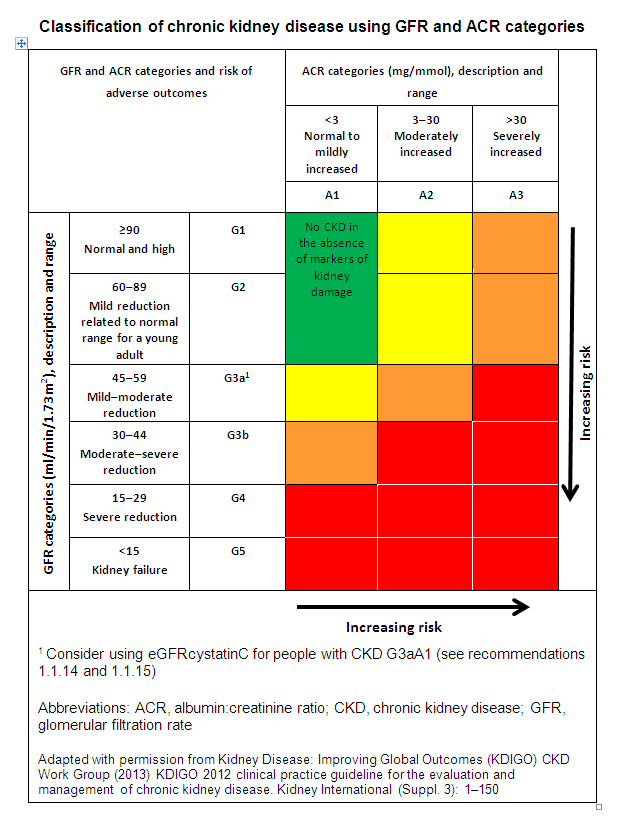
The higher the stage of CKD, and worse the proteinuria, the more frequent monitoring patients require. This helps to identify and manage complications, and plan for renal replacement therapy (RRT; i.e. dialysis and/or transplantation). The higher the ACR and lower the eGFR, the more at risk a patient is of adverse outcomes. These include CKD progression, acute-on-chronic kidney injury, all-cause mortality, and cardiovascular events.
Causes
CKD is not a diagnosis. It is a syndrome with causes. As stated above, it is your role to ascertain the underlying pathology (cause) as this guides treatment.
There are 7 groups of causes:
- Unknown (often with small kidneys). This is the ‘cause’ in 30% of patients
- Diabetes (mainly type 2). This is the cause in 20% of patients, i.e. the second commonest
- Renovascular disease. This mainly occurs in older people who are smokers or ex-smokers; and/or people with congestive heart failure as well. Then its is often called ‘cardiorenal syndrome’
- Obstructive nephropathy. This means a blockage in the drainage system of the kidneys – i.e. ureters, bladder, or prostate (in men). It is usually due to prostatic disease in men, or pelvic cancer in women. Kidney stones can cause it, but rarely (unless the patient has one kidney)
- Glomerulonephritis / vasculitis. These are ‘autoimmune’ diseases. There are 7 types, which can present in many different ways
- Tubulointerstitial disease. There are 3 subgroups: autoimmune, drugs and infection (e.g. reflux nephropathy). The drug causes include non-steroidal anti-inflammatory drugs (NSAIDs; if they are used long-term, especially at high doses), lithium, ciclosporin and tacrolimus
- Polycystic kidney disease. This is the only common familial (inherited) cause.
Note. Simple primary (essential) high blood pressure, and simple recurrent urinary tract infections (UTIs), do not cause CKD. This is a common false belief. You should find another cause in a patient with CKD and simple high BP or UTIs.
The causes of CKD in children and young adults are different.
Ageing and the kidney
Approximately 1 million nephrons are present in each kidney from birth. These nephrons carry out glomerular filtration and tubular reabsorption, allowing the kidney to perform its normal functions (e.g. volume regulation, acid-base balance).
As we age, there is a progressive loss in renal mass, and a number of structural changes occur (e.g. glomerulosclerosis) leading to a decline in renal function. Many doctors think following a peak at the end of the third decade of life, there is an estimated annual decline of 1 mL/min/year in eGFR (though this ‘fact’ is controversial).
Clinical features
Patients are generally asymptomatic with mild-moderate CKD (CKD1-3), but start to develop non-specific symptoms at more advanced stages (e.g. CKD4 with eGFR < 30ml/min).
On examination, it is important to look for evidence of an underlying cause of CKD – e.g. large bilateral abdominal masses could suggest PCKD; a palpable bladder is suggestive of obstructive nephropathy; and epigastric or femoral bruits are suggestive of renovascular disease) .
Symptoms
- Frequently asymptomatic in early stages
- Anorexia and nausea
- Fatigue and weakness
- Shortness of breath or oedema
- Muscle cramps
- Other: pruritus, pain, poor sleep and concentration, restless legs.
Signs
- Pallor (secondary to anaemia)
- Hypertension
- Fluid overload
- Rashes (vasculitis)
- Excoriation marks
- Peripheral neuropathy
- Lindsay’s nails (‘half-and-half nails’)
These are distal brown transverse band that can be seen in chronic kidney disease (CKD). They are caused by increased pigment deposition.
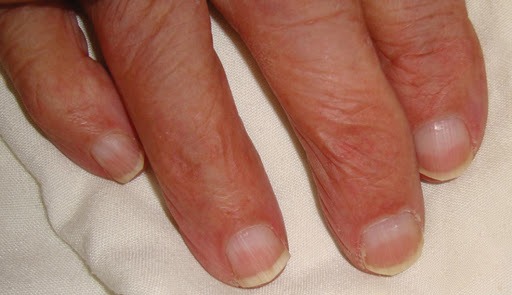
Lindsay’s nails (‘half-and-half nails’)
In severe renal impairment, patients may develop high urea levels that can lead to number of manifestations. These include: ‘uraemic encephalopathy’ (i.e. seizures, confusion, low consciousness); pericarditis (e.g. pericardial effusion and clinical rub on auscultation) and/or pleuritis (with rub), or both; and defective platelet function (causes bruising), amongst others.
Note. If the urea is over 50 mmol/L, you need a good reason not to dialyse the patient.
Investigation
Investigations are used to help diagnose, monitor and assess for complications of CKD.
Urine
- Urine dipstick: heavy proteinuria is suggestive of glomerular disease
- Urine microscopy – casts
- Urinary albumin:creatinine ratio (uACR)
Note. uACR is used instead of a urinary protein:creatinine ratio (uPCR) because of its greater sensitivity at a lower level of proteinuria.
Bloods
- FBC
- U&E (+ eGFR)
- Glucose
- Bone biochemistry
- Bicarbonate
- LFTs
- Lipid profile.
Renal screen
- Anti-nuclear antibody (ANA) – Systemic Lupus Erythematosus (SLE)
- Anti-neutrophil cytoplasmic antibody (ANCA; with PR3 and MPO) – vasculitis
- Anti-glomerular basement membrane (anti-GBM) antibody – Goodpasture’s Disease
- Anti phospholipase A2 receptor (PLA2R) antibody – primary membranous nephropathy
- Complement C3/4 – low in SLE
- Double-stranded DNA (DsDNA) – SLE
- Serum electrophoresis – paraprotein suggestive of myeloma (and other blood disorders)
- Immunoglobulins (IgG, A and M) – e.g. IgA high in IgA nephropathy
- Serum free light chains (SFL) – myeloma (and other dysproteinaemias)
- Hep B/C, HIV – viral associated GNs (membranous GN with Hep B, mesangiocapillary GN with Hep C, FSGS with HIV).
Imaging
- Renal ultrasound (US) – all patients with CKD3B or worse should have an US. Full stop. And some patients with CKD1-3A
- Nuclear medicine scans, or CT or MR angiography – in a few patients. Renal angiograms are now rarely performed.
A renal ultrasound scan should be considered in patients with visible (or persistent non-visible) haematuria, or a family history of PCKD.
Special
Renal biopsy is required to identify intrinsic (renal) causes of CKD, especially glomerulonephritis and tubulo-interstitial nephritis.
Note. Patients with evidence of macrohaematuria in the absence of infection (and no obvious GN), should be investigated initially (and rapidly) by a urologist for urinary tract malignancy. Once that has been excluded, a nephrologist should see the patient.
GFR/creatinine and uACR monitoring
This is highly variable depending on the level of GFR and rate of decline.
CKD1-2. Risk factors for CKD. Creatinine – normal (< 120 mcml/L)
- eGFR/creatinine should be measured every 12 months
CKD3A. Mild CKD. Creatinine – normal or <150 mcmol/L
- eGFR/creatinine – every 6 months
CKD3B. Mild CKD (but worse than CKD3A). Creatinine usually 150-200 mcmol/L
- eGFR/creatinine – every 4-6 months
- Urinary ACR (uACR) – if monitoring the protein level in the urine is important in your disease – every 6 months
CKD4. Moderate CKD. Creatinine usually 200-400 mcmol/L
- eGFR/creatinine – every 2-4 months
- uACR, if needed – every 4 months
CKD5. Severe CKD (approaching dialysis). Creatinine >400 mcmol/L
- eGFR/creatinine every 1-2 months.
This is only rough guidance from NICE concerning the minimum monitoring for patients with CKD. Some patients at high risk of progression or adverse outcomes (e.g. rapidly deteriorating renal function) may need their kidney function checked more frequently.
Note. When on dialysis, and when stable, the frequency of monitoring can go down, eventually to every 4-6 months.
Management and treatment – 4 goals
The 4 goals of CKD management are:
- To treat the underlying cause
- Prevent or slow progression – i.e. renoprotective therapy
- Treat associated complications
- Plan for RRT.
Renoprotective therapy
Renoprotective therapy is aimed at slowing the progression of CKD, independent of the aetiology. It is centered around tight blood pressure control and reducing proteinuria.
A standard BP target of <140/90 mmHg or below, should be aimed for in patients with CKD. This is broadly in line with the NICE guidelines on hypertension. However, in patients with diabetes, or if the ACR is > 70 mg/mmol, then a tighter blood pressure control should be targeted (< 130/80 mmHg).
In adults with CKD, hypertension and an ACR ≤ 30 mg/mmol, the advice on choice of anti-hypertensive should be line with the NICE guidelines on hypertension. In patients with more significant proteinuria, the ideal blood pressure agent is an ACE inhibitor or angiotensin receptor blocker (ARB; both renin-angiotensin system antagonists). These drugs are both antihypertensive and antiproteinuric.
Note. But do not routinely offer a renin-angiotensin system antagonist in CKD if the pre-treatment potassium is > 5 mmol/L.
Sodium–glucose cotransporter 2 inhibitors (SGLT2is)
SGLT2is (e.g. dapagliflozin) are commonly used hypoglycaemic agents for patients with type 2 diabetes mellitus. These medications block SGLT2 receptors expressed on the proximal convoluted tubules of nephrons. In doing so, they prevent reabsorption of filtered glucose (i.e. its antidiabetic effect).
However, inhibition has added benefits on renal haemodynamics, including decreasing renal intraglomerular pressure and inhibiting the renin-angiotensin-aldosterone system. This means they can have beneficial effects for patients with proteinuric CKD and heart failure, as well as diabetes.
Hence an SGLT2i (like Dapagliflozin) is now recommended in patients with CKD, added to an optimal dose of an ACE/ARB (unless contraindicated), who have:
- eGFR >25 and <60 ml/min, AND
- Type 2 diabetes mellitus, OR
- Urinary ACR ≥ 25 mg/mmol.
This is a complicated area. These two papers may help you:
- https://www.cardioaragon.com/wp-content/uploads/SGLT2-inhibitors-the-statins-of-the-21st-century.EHJ_.2022.pdf
- https://link.springer.com/article/10.1007/s11892-021-01442-z.
Other therapies
- HMG-CoA reductase inhibitors (statin therapy): prescribed in line with NICE recommendations
- Smoking cessation
- Antiplatelets for secondary prevention of CVS disease.
Specific treatments
- In obstructive nephropathy, a urinary catheter +/- nephrostomies may be required
- In some autoimmune conditions, immunosuppression is necessary, with drugs such as prednisolone, cyclophosphamide, ciclosporin/mycophenolate, and rituximab. A nephrologist will decide if these are required.
Complications
A number of important complications develop as a consequence of CKD. These include anaemia, hyperkalaemia, mineral and bone disorders, fluid overload and acidosis.
Anaemia
A normocytic normochromic anaemia is typical of CKD. This anaemia is normally multifactorial. But a significant factor in advanced CKD is a reduction in the production of erythropoietin (EPO), the hormone that drives erythropoiesis.
It is still important to assess patients for other potential causes of anaemia (e.g. iron-deficiency, folate deficiency), which can subsequently be corrected. The mainstay of treatment for anaemia in CKD is an erythropoietin-stimulating agent (ESA); such as Darbepoetin alfa (e.g. Aranesp), or a hypoxia-inducible factor–prolyl hydroxylase (HIF-PH) inhibitor, like Roxadustat (given as a tablet three times a week).
In order for EPO therapy to be effective, patients need to have adequate iron stores that should be regularly checked and replaced. In fact, initiation of EPO is not recommended until iron-deficiency has been managed.
Blood transfusions should be avoided where possible in patients who are being considered for renal transplantation. This is because of the risk of sensitisation (i.e. development of antibodies) to human leucocyte antigens (HLA).
When the haemoglobin (‘HB’; marker of anaemia) falls below 100 g/L, EPO should be considered. The target haemoglobin concentration in patients with CKD-associated anaemia is 100-120 g/L.
White count and platelets are typically normal in CKD. There may be a bleeding tendency due to thrombasthenia (platelet dysfunction).
Hyperkalaemia
The ability of the kidneys to maintain adequate acid-base homeostasis and electrolyte balance diminishes with worsening renal function. Also, many medications, including NSAIDs, ACE/ARBs and potassium-sparing diuretics, may cause or worsen hyperkalaemia. Furthermore, uncontrolled metabolic acidosis may worsen potassium handling.
Acute rises in potassium should be managed as a medical emergency. This involves stabilisation of the myocardium (with calcium gluconate) and driving potassium into the intracellular compartment (with insulin/dextrose). Chronic elevations in serum potassium can be managed with low potassium diets, potassium-binding resins and correction of acidosis. Dialysis may eventually be required.
Renal bone disease (RBD) or renal osteodystrophy
In CKD, disorders of mineral and bone metabolism reflect a complex spectrum of pathology that results from abnormal calcium and phosphate handling. In health, the kidneys have an important role in the maintenance of calcium homeostasis. They activate vitamin D (calcitriol), a fat-soluble vitamin important for absorption of calcium from the gastrointestinal tract. They are also involved in the reabsorption of calcium and excretion of phosphate.
Reduced kidney function (usually associated with CKD4 or worse, i.e. GFR < 30ml/min) leads to hypocalcaemia, hyperphosphataemia and hyperparathyroidism (secondary hyperparathyroidism). These biochemical abnormalities eventually lead to bone pathology (e.g. adynamic bone disease, osteomalacia, osteoporosis and osteitis fibrosa cystica). The term ‘renal bone disease (RBD) or ‘renal osteodystrophy’ is used to describe this type of bone pathology seen in CKD.
The treatment of RBD requires management of the underlying biochemical abnormalities:
- Hypocalcaemia: phosphate binder (e.g. calcium acetate) and vitamin D (e.g. alfacalcidol)
- Hyperphosphataemia: dietary phosphate restriction and calcium acetate
- Hyperparathyroidism: calcimimetics (e.g. cinacalcet) or surgery (parathyroidectomy).
Fluid overload
In the presence of significantly reduced GFR, the kidneys are unable to control fluid volume. This leads to hypervolaemia and patients may have oedema (ankle, sacral, pulmonary), ascites, raised JVP, gallop rhythm and bilateral pleural effusions.
Note. Heavily nephrotic patients usually have pleural effusions (and sometimes peri-orbital oedema) rather than pulmonary oedema.
Fluid overload can be managed with a combination of fluid restriction, reduced sodium intake and the use of oral diuretics (e.g. furosemide). Dialysis may eventually be required.
Acidosis
Patients with CKD retain hydrogen ions because of abnormalities in their acid-base homeostasis. Acidosis may also exacerbate hyperkalaemia, further compounding the metabolic abnormalities seen in CKD.
Acidosis is characterised by a low pH and low bicarbonate levels. Oral sodium bicarbonate therapy should be considered in patients with an eGFR < 30 ml/min (CKD4 or worse), and bicarbonate level < 20 mmol/L.
Indications for dialysis
If the following complications of CKD occur, and are refractory to medical therapy, dialysis is required:
Absolute indications
- Hyperkalaemia
- Fluid overload.
Relative indications
- Metabolic acidosis
- Hypertension
- Uraemic complications (e.g. encephalopathy, bleeding, pericarditis).
Renal replacement therapy (RRT)
Haemodialysis, peritoneal dialysis and renal transplant are the 3 forms of RRT used for ESRF. Supportive care – i.e. not having RRT – should also be considered for older, frailer patients.
Haemodialysis
Haemodialysis involves the removal of waste products and other substances by passing blood through a dialysis machine. Blood comes into contact with a semi-permeable membrane, which contains the dialysate on the other side. Substances may then diffuse between the two fluids (blood and dialysate).
Haemodialysis requires intravenous access in the form of an arteriovenous fistula (or graft) or tunnelled line. Patients usually have haemodialysis for 3-5 hours, 2-3 times a week.
Peritoneal dialysis
Peritoneal dialysis is achieved by using the peritoneal cavity as the primary site of ultrafiltration. A catheter (e.g. Tenckhoff catheter) is inserted into the abdominal cavity, which allows the infusion of the dialysate (usually a glucose solution).
The dialysate then dwells in the abdomen using the peritoneum as a semi-permeable membrane for the transfer of waste products. The dialysate can then be removed after a certain amount of time and the procedure repeated.
Peritoneal dialysis can be achieved by continuous ambulatory peritoneal dialysis (CAPD) where 4 exchanges (of 2 litres of dialysate) are made manually each day; or by automated peritoneal dialysis (APD) where 8-10 litres are exchanged overnight (10-12 hours) by a machine, when the patient sleeps.
Renal transplant
Renal transplant is considered the gold-standard for many patients with ESRF. Transplantation may be from living donors or non-living donors. It is now possible to transplant across the ABO (blood group) and HLA barriers. Non-living donors include donors after cardiac death (DCD) and donors after brain death (DBD).
Renal transplantation requires long-term immunosuppressive therapy to stop the recipient’s immune system from ‘attacking’ the donor tissue.
Despite the overt success of transplantation, it can be associated with a number of complications including graft rejection, complications from immunosuppressive agents (e.g. malignancy, infection) and disease recurrence.
Kidneys from deceased and living donors last about 10 and 15 years respectively. Hence younger patients may require more than one transplant in their lives.
Supportive care
This means not having dialysis or a transplant, despite the patient being in Stage 5 CKD. It does not mean ‘no treatment’. Diuretics, EPO, vitamin D and anti-hypertensives should be given as normal. Good communication with the patient’s GP and community-based supportive care nurses if required, are essential.
Summary
We have described CKD – the basics (for health professionals). We hope you have found it helpful.
Other resource
https://www.youtube.com/watch?app=desktop&v=vZBDHh3gzus
Last Reviewed on 3 July 2024
